6 Tri-Cities Locations
Quality, Patient-Centered Health Care
Quality Health Care for All
We are dedicated to the communities we serve, bringing together a unified team that delivers the highest-quality health care to every person, every time.
We Believe Everyone Deserves Quality Health Care
We provide exceptional health care services to everyone, regardless of their economic or legal status. We serve patients with or without insurance and offer a sliding fee scale to those who qualify.
We are a Federally Recognized Public Health Institution
As a Health Center Program grantee and a deemed Public Health Service, we provide quality, patient-centered healthcare services and access to care for all.
We Offer Whole Body Care for People of All Ages
With primary care experts for pediatrics through adulthood and specialists to cover everything from dental health to diabetes management, our unified team of providers work together to provide comprehensive care from head to toe.
TCCH in the Community
We are excited to share with you the latest updates and activities happening within our community as we continue our mission to promote health and wellness for all.

TCCH Celebrates National Health Center Week
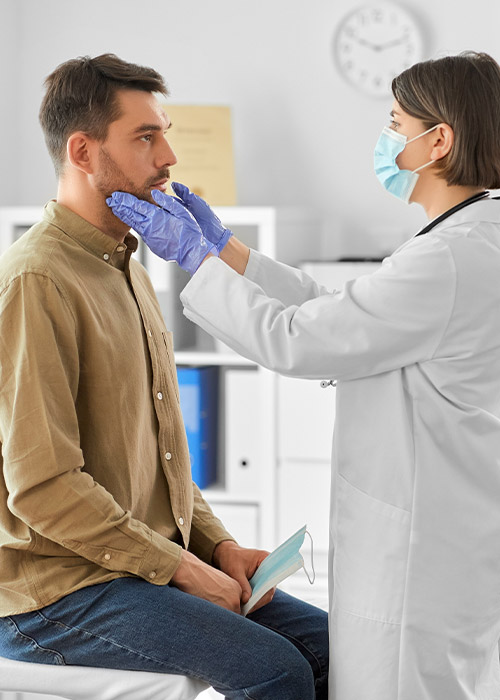


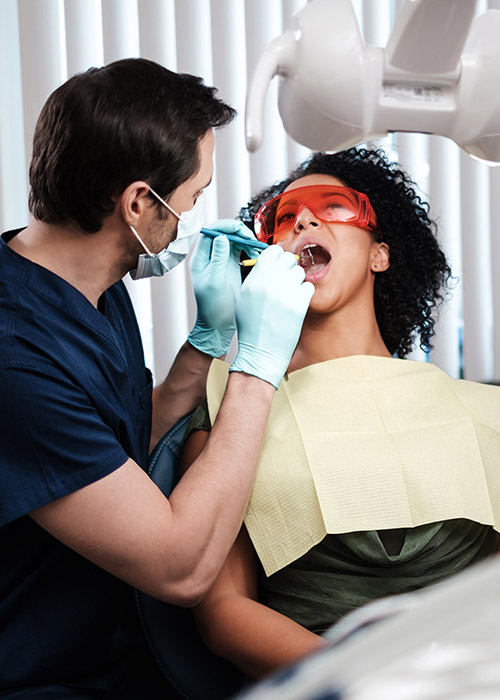
Our Services
Through our network of clinics, TCCH delivers comprehensive primary care, behavioral health, and dental services, helping to improve the health and well-being of individuals and families in the area.
FSHCAA Deemed Health Center
Tri-Cities Community Health (TCCH) is a Health Center Program grantee under 42 U.S.C. 254b, and a deemed Public Health Service employee under 42 U.S.C. 233(g)-(n) providing quality, patient-centered, health care services and access to care for all.






